生物治疗全国重点实验室
Brief Introduction of State/National Key Laboratory of Biotherapy and National Research and Development Platform for Novel Drug
State/National Key Laboratory of Biotherapy (SKLB) was established by the Ministry of Sciences and Technology in China in 2005, and became one of the National Research and Development (R&D) Platforms for Novel Drugs founded by the Ministries of Sciences and Technology as well as Public Health in September 2008. It is also supported by National Collaborative Innovation Program and became the National Collaborative Innovation Center for Biotherapy in April 2013. The Platform is located in the High Tech Zone of Chengdu and the Medical Campus of Sichuan University. The overall space is nearly 70,000 square meters and is still under intensive growth and construction. SKLB also has rich clinical resources of West China Hospital, which has 4,300 beds.
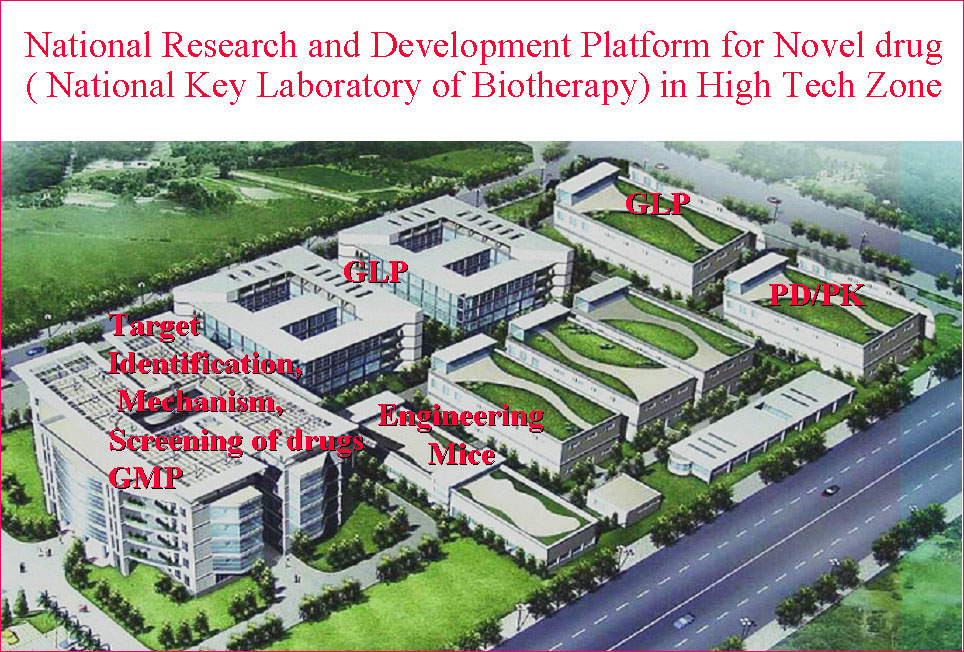
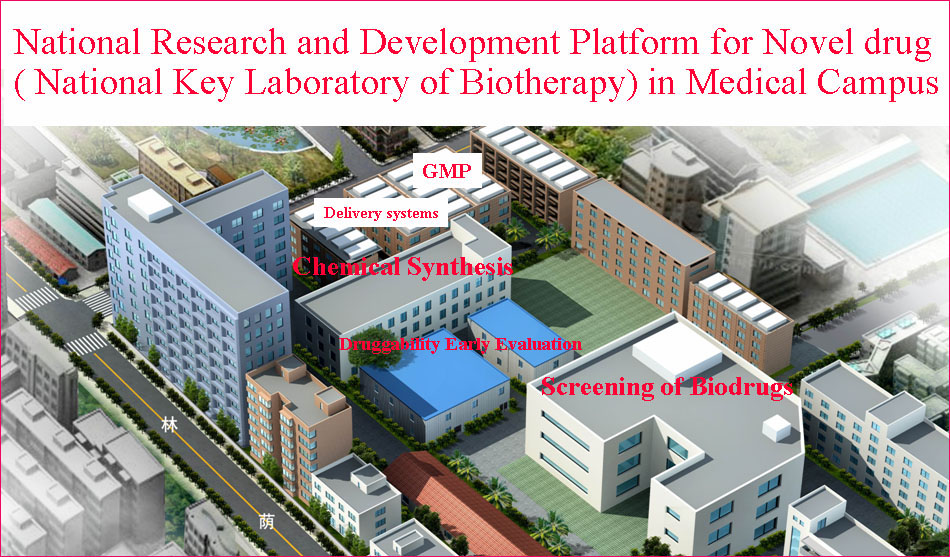
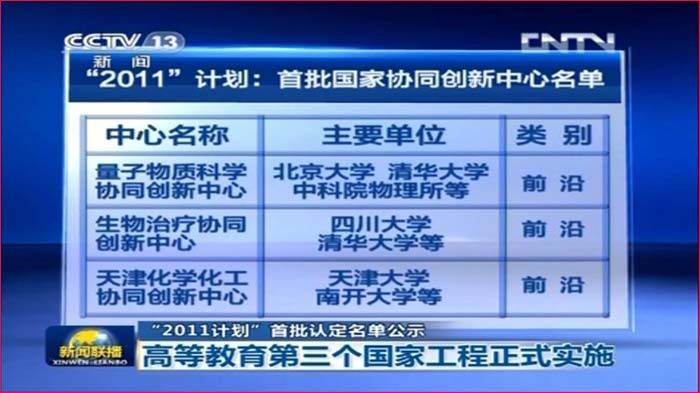
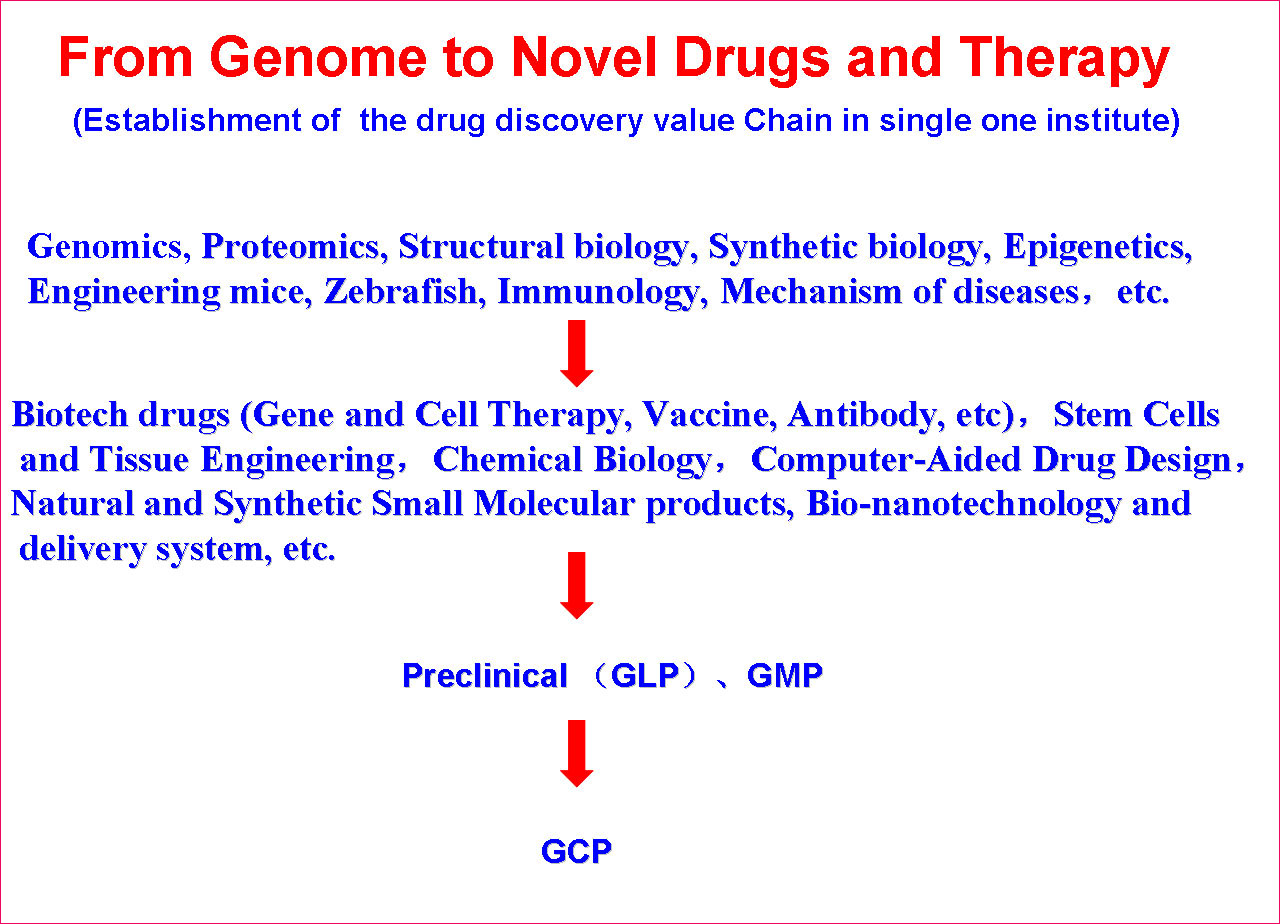
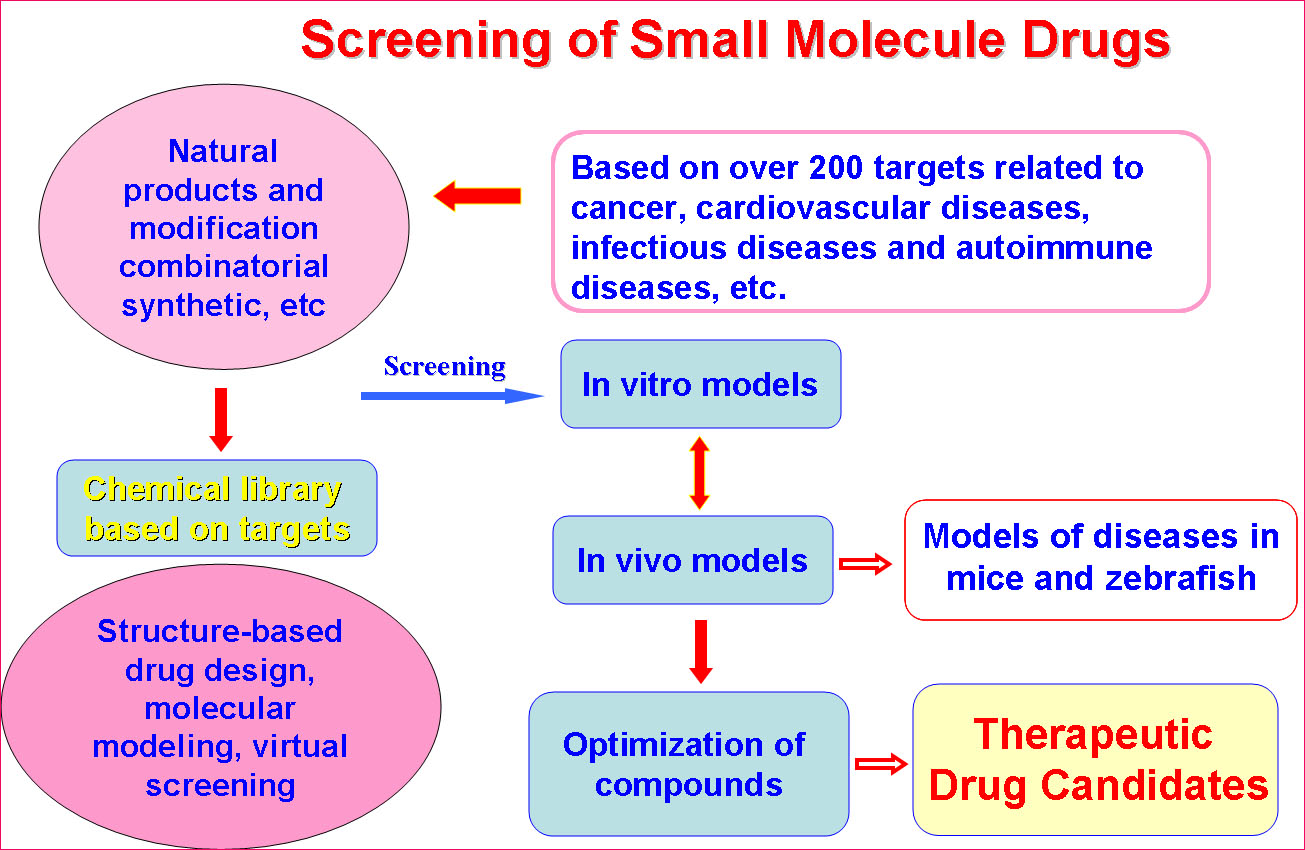
SKLB is becoming a highly-regarded, comprehensive and multidisciplinary research center in China. Through the seamless integration of basic research, preclinical development, translational and clinical medicine, an efficient and fully integrated technology chain for the discovery and development of innovative drug candidates has been established in a single institute. Part of the mission of this Platform is to improve the treatment of major human diseases such as cancer, cardiovascular diseases, obesity, diabetes mellitus, infectious diseases (hepatitis, AIDS, and tuberculosis etc), inflammatory diseases, neurological disease, as well as chronic autoimmune diseases. Focusing on more than 200 important targets, hundreds of projects for basic research and biologic drugs (such as gene and cell therapy, vaccines, and monoclonal antibodies, recombinant proteins, etc), synthetic small molecule drugs and small molecule natural products are ongoing.
The Platform boasts a prominent faculty of nearly 100 well-funded full professors, associate professors and assistant professors, with diverse disciplines such as biotechnology, medicine, immunology, pharmacology, chemistry, formulations, drug delivery, and material sciences. Every year the faculty can obtains competitive grants from the government, including the National High Technology Research and Development Program (“863” Program) or the Major State Basic Research Development Program (“973” program). Over 300 research papers are published in peer-viewed international journals every year, including such top journals as New Engl J Med, Nat Rev Drug Discovery, Dev Cell, Nat Chemical Biol, Nature Med, Mol Cell, Proc Natl Acad Sci USA, Cancer Res, and Lancet Neurol. To date, the Platform has licensed over 50 patents to the commercial sector throughout China. Furthermore, 45 potent candidate drugs thus far have been transferred to over 30 pharmaceutical companies for commercial development.
The Platform also provides both key technological services and integral solutions in R&D for other institutions and companies, including:
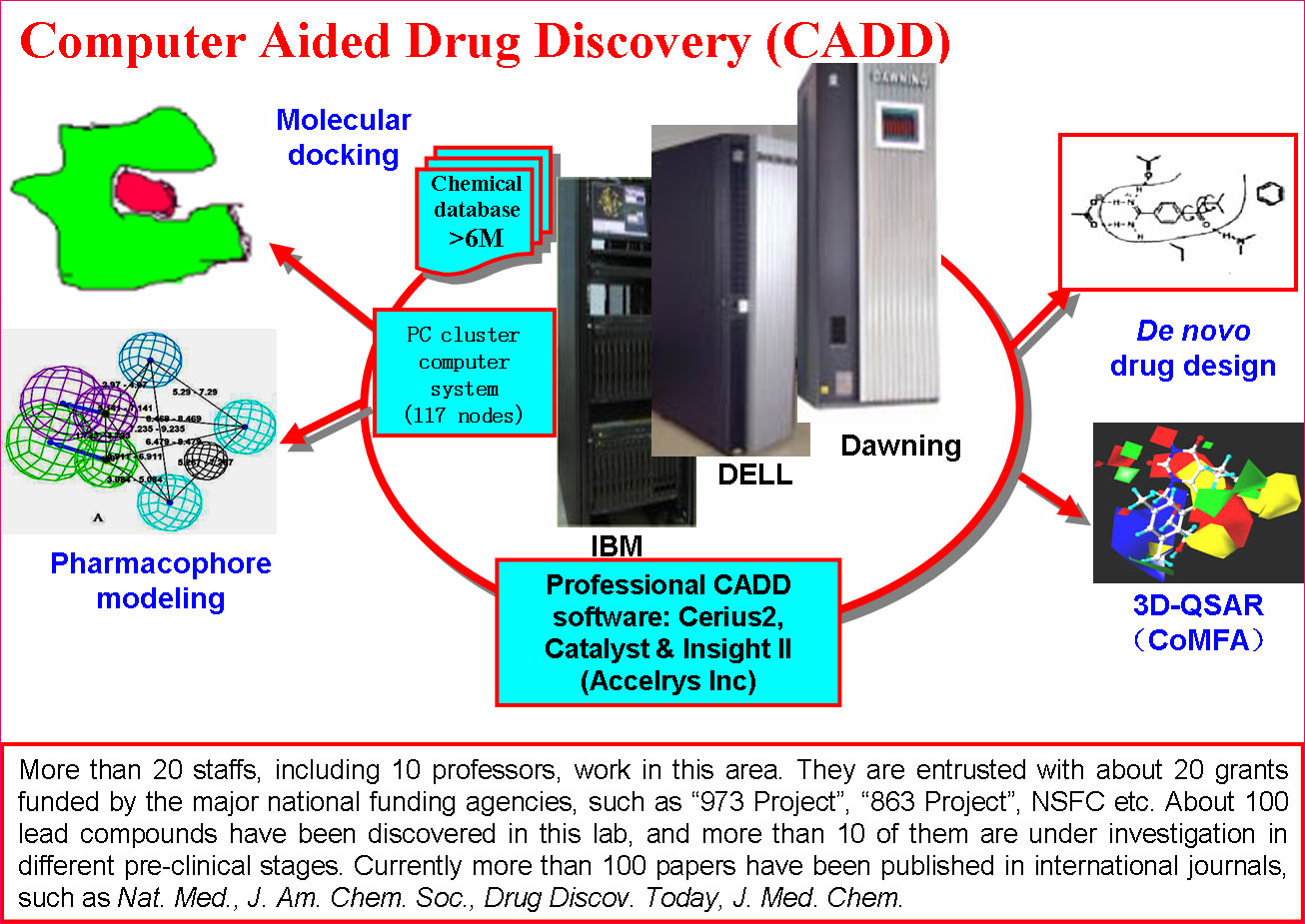
(1) Target Identification and Drug DiscoveryThe Platform has established multiple sub-platforms, such as Proteomics and genomics, structural biology, epigenetics, immunology, molecular cell biology,developmental biology,the mechanism of diseases, biopharmaceuticals R&D, gene and cell therapy, monoclonal antibody and vaccine, stem cell/tissue engineering, engineering mice and zebrafish, drug delivery and nanotechnology, computer-aided drug design, chemical biology, screening and synthesis of small molecular drugs, and the separation and purification of active natural products, especial in the development of novel therapeutic approaches. 

 (2) Pilot-Scale Production Six GMP-level facilities for pilot-scale production of adenoviral vectors, vaccines, DNA plasmids, recombinant proteins and nanoparticles have been established. For example, the pilot-scale production of DNA plasmids provides 5000-8000 doses for early phase clinical trials.
(2) Pilot-Scale Production Six GMP-level facilities for pilot-scale production of adenoviral vectors, vaccines, DNA plasmids, recombinant proteins and nanoparticles have been established. For example, the pilot-scale production of DNA plasmids provides 5000-8000 doses for early phase clinical trials.  (3) Pharmacodynamics & Pharmacokinetics Three separate animal facilities can maintain up to 60,000 mice. At full capacity, 300 drug candidates can be evaluated at the same time. To date, there have been over 100 different preclinical disease models established at these facilities.
(3) Pharmacodynamics & Pharmacokinetics Three separate animal facilities can maintain up to 60,000 mice. At full capacity, 300 drug candidates can be evaluated at the same time. To date, there have been over 100 different preclinical disease models established at these facilities.

(4) Pre-Clinical Safety Evaluation The animal center has currently obtained international“AAALAC”accreditation. The GLP facility can maintain up to 500 monkeys. Various toxicity studies including single dose toxicity, repeated dose toxicity, reproductive and developmental toxicity, genotoxicity, carcinogenicity, immunogencity, and pharmacokinetic/toxicokinetic (PK/TK) analyses are all performed.  (5) Clinical trials Clinical trials testing gene and cell therapy, cytokines and anti-cytokines, monoclonal antibodies, chemotherapy and targeted small molecule drugs are conducted in the clinical units of West China Hospital with 4300 beds.
(5) Clinical trials Clinical trials testing gene and cell therapy, cytokines and anti-cytokines, monoclonal antibodies, chemotherapy and targeted small molecule drugs are conducted in the clinical units of West China Hospital with 4300 beds.  (6) Graduate programs The platform also is an educational center. Every year the platform enrolls about 150 Ph.D and 100 M.S students for different academic programs, as well as nearly 100 M.S. graduate students for pharmaceutical engineering programs.
(6) Graduate programs The platform also is an educational center. Every year the platform enrolls about 150 Ph.D and 100 M.S students for different academic programs, as well as nearly 100 M.S. graduate students for pharmaceutical engineering programs.
研究方向简介
生物治疗是现代生物技术与临床医学等多学科交叉融合而形成的针对人类重大疾病,如肿瘤、心脑血管疾病、感染性疾病、自身免疫性疾病等进行治疗研究的新兴领域。生物治疗研究范围非常广泛,主要包括针对重大疾病的生物技术药物、基因治疗、免疫治疗(疫苗治疗、抗体治疗、细胞因子治疗等)、调节血管生成治疗、干细胞及组织工程治疗、诱导分化及凋亡治疗等相关的基础研究、应用基础研究、关键技术及产品研发等。
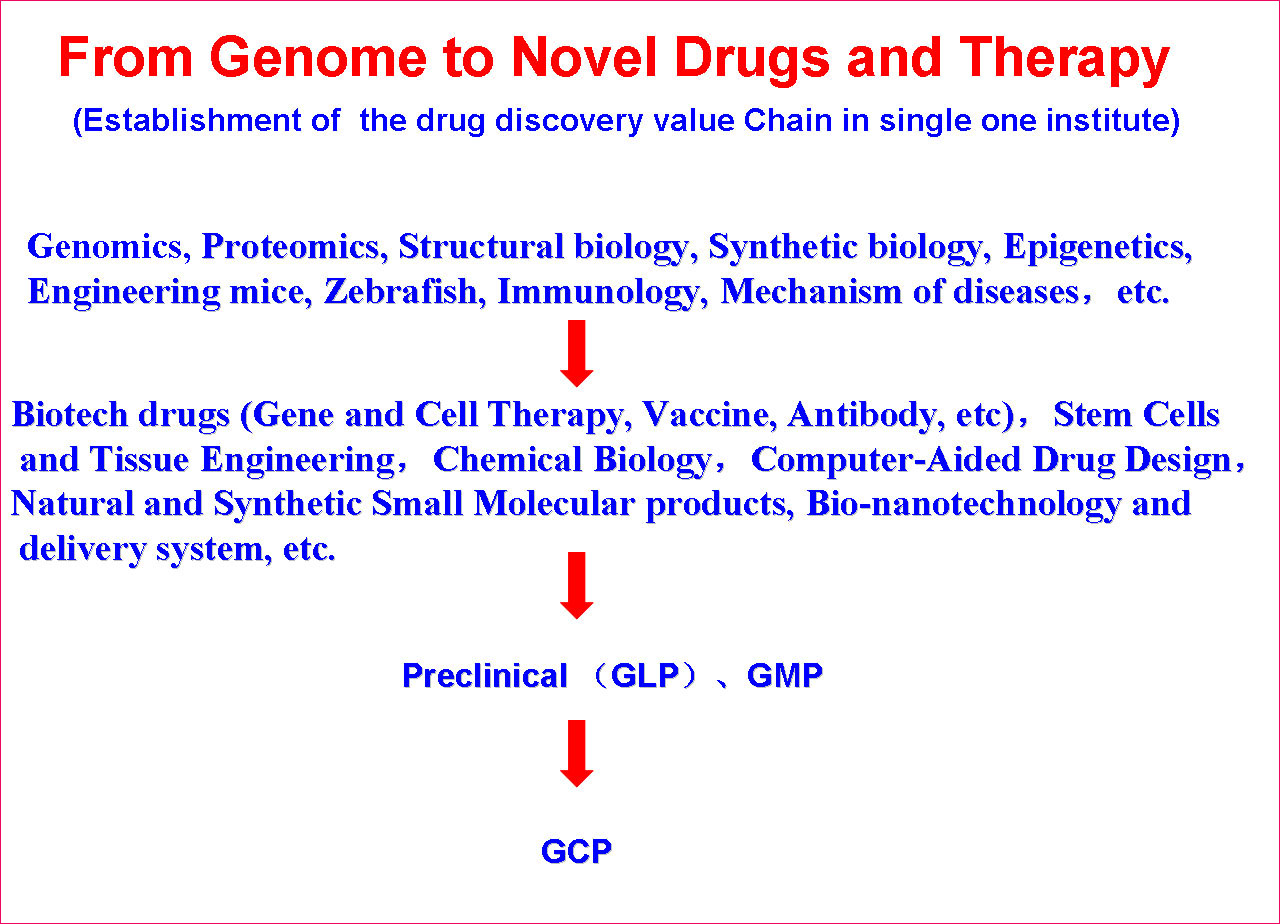
生物治疗国家重点实验室的总体定位是将现代生物技术、临床医学、免疫学、药物学、纳米生物技术、化学、材料学等多学科进行交叉与融合,致力于人类重大疾病生物治疗的基础研究、应用基础研究、关键技术及产品研发,建设国内一流、国际知名的生物治疗实验室,促进医药生物技术的发展。目前,已建立了从基因发现到药物研发及临床治疗等一系列关键技术平台,如基因组学、蛋白质组学、模式生物(基因工程小鼠、斑马鱼与非洲爪蟾);生物技术药物、基因治疗、干细胞与组织工程、纳米生物技术、化学生物学、天然小分子药物、计算机辅助药物设计等药物研发关键技术平台;已建成了生物技术药物及天然药物的GMP中试生产车间、国家新药临床前评价中心和国家新药临床试验基地(生物治疗)。形成了以下5个主要研究方向:
1. 肿瘤、感染性疾病等重大疾病生物治疗研究:重点研究针对肿瘤、感染性疾病(如肝炎、HIV、等)、心血管疾病、自身免疫疾病、呼吸系统疾病、遗传病等的生物治疗。
2.功能基因组学及蛋白质组学研究:重点发展和完善基因组学、蛋白组 学和生物信息学的研究平台,结构生物学、表观遗传学、建立模式生物、基因敲除平台,重点进 行重要致病基因的克隆、鉴定及突变分析与基因多态性的研究;免疫应答分子机理研究;信号传导与细胞凋亡研究等,为人类重大疾病生物治疗奠定理论基础及提供新的靶分子。
3.干细胞与组织工程研究:重点研究人胚胎干细胞、骨髓基质干细胞、造血干细胞、肌肉干细胞和脂肪干细胞等;研究工程化组织替代(包括骨、软骨、肌腱、皮肤、角膜)、消化及泌尿管道的再生与修复(主要采用工程化组织替代及诱导组织再生技术,重点是食管、膀胱、尿道、胆道);心脏病的治疗(包括心肌缺血的再生,组织工程人工心瓣,异位节律的重建,冠心病的补片材料)。
4.化学生物学/化学基因组学及纳米生物技术研究:重点研究特异性小分子的设计与合成、小分子与大分子间的相互作用及生物活性、小分子靶向抗肿瘤药物的开发等;研究靶向式药物释放(微纳米)载体(膜)技术、可控降解药物胶囊材料与系统、研究靶向药物与新型给药系统。
5.新药临床前评价和新药临床试验(生物治疗):主要开展的生物治疗 临床研究有造血干细胞移植、细胞因子治疗、抗体治疗以及多种生物化疗方案等。该研究中心作为国家新药临床试验基地,已承担了多项抗肿瘤生物技术新药的临床试验研究。

 西南财经大学
西南财经大学 西南交通大学
西南交通大学 四川师范大学
四川师范大学 电子科技大学
电子科技大学 四川农业大学
四川农业大学









